Production of N-containing compounds from plastic wastes by fast pyrolysis
Recently, Dr. Lujiang Xu and Prof. Zhen FANG have developed a catalytic pyrolysis process with ammonia for direct conversion of polyethylene terephthalate (PET) to produce N-containing compounds such as Terephthalonitrile (TPN).
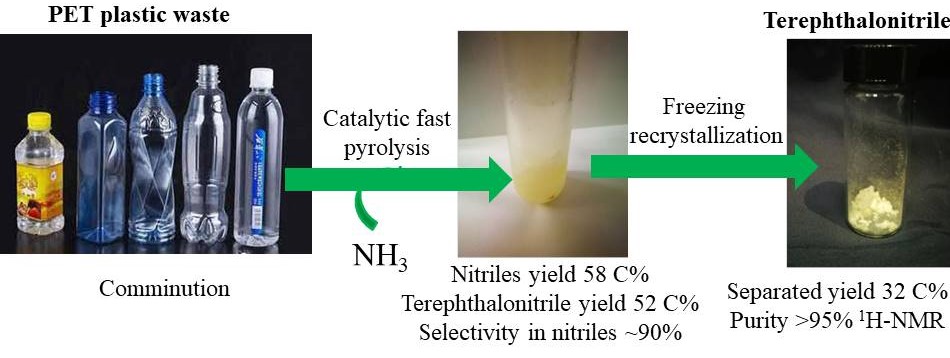
Production of terephthalonitrile from polyethylene terephthalate over γ-Al2O3 based catalysts by fast pyrolysis with ammonia (γ-Al2O3催化快速热解氨化聚对苯二甲酸乙二醇酯PET塑料制备对苯二甲腈)
The optimal condition for producing TPN was over 1 g γ-Al2O3-2wt% catalyst at 500 ºC under carrier gas (50% NH3 and 50% N2) with yield of nitriles and TPN of 58.1 and 52.3 C%, respectively. The selectivity of TPN in the nitriles was around 90%. Meanwhile, a bit of aromatics, benzonitrile, acetonitrile were also produced as by-products with the total yields of less than 3 C%. The catalyst deactivated slightly after 5 cycles. Possible reaction routes were proposed and it was found that terephthalic acid, benzoic acid, related esters and amides were the major intermediates from PET to nitriles. Acetonitrile could be produced from acetaldehyde and its corresponding imines. In addition, 32.1 C% TPN with high purity (> 95%) was obtained via freezing recrystallization.
The carboxyl group in PET plastic was efficiently utilized for the production of terephthalonitrile and benzonitrile by controlling Ca(OH)2/γ-Al2O3 catalysts and pyrolysis parameters (e.g. temperature, residence time, ammonia content). The best conditions are selected as 2% Ca(OH)2/γ-Al2O3 (0.8 g), 500 ºC under pure ammonia with 58.3 C% terephthalonitrile yield and 92.3% selectivity in nitriles. In addition, 4% Ca(OH)2/ Al2O3 was suitable for producing benzonitrile. With catalyst dosage of 1.2 g, residence time of 1.87 s, pyrolysis temperature of 650 ºC and pure ammonia (160 mL/min carrier gas flow rate), the yield and selectivity of benzonitrile were 30.4 C% and 82.6%, respectively.
Related results were published:
1. LJ Xu, XW Na, LY Zhang, Q Dong, GH Dong, YT Wang, Zhen Fang*, Selective Production of Terephthalonitrile and Benzonitrile via Pyrolysis of Polyethylene Terephthalate (PET) with Ammonia Over Ca(OH)2/Al2O3 Catalysts, Catalysts, 9, 436; doi:10.3390/catal9050436 (2019).
2. LJ Xu, LY Zhang, H Song, Q Dong, GH Dong, X Kong, Zhen Fang*, Catalytic Fast Pyrolysis of Polyethylene Terephthalate Waste Plastic for the Selective Production of Terephthalonitrile under Ammonia Atmosphere, Waste Management, 92, 97–106 (2019).
------------------------------------------------------------------------------------------
利用塑料废弃物快速热解制备含氮化合物
最近,徐禄江博士和方真教授开发了一种氨催化热解法,将聚对苯二甲酸乙二醇酯(PET)塑料直接转化为对苯二甲腈(TPN)等含氮化合物。
在载气(50%NH3和50%N2)和温度500℃下,制备TPN的最佳条件为1 g γ-Al2O3-2wt%催化剂,腈类化合物和TPN的收率分别为58.1%和52.3%。TPN在腈类中的选择性约为90%。同时,还生产了少量芳烃、苯甲腈、乙腈等副产品,总收率小于3%。经过5个循环后,催化剂略微失活。作者提出了可能的反应路线,发现对苯二甲酸、苯甲酸、相关酯和酰胺是从PET到腈的主要中间体。乙醛及其亚胺可制得乙腈。此外,通过冷冻再结晶得到了32.1%的高纯度TPN(>95%)。
作者进一步通过控制Ca(OH)2/γ-Al2O3催化剂和热分解参数(如温度、停留时间、氨含量),有效利用PET塑料中的羧基来生产对苯二甲酸和苯甲腈。选择2%Ca(OH)2/γ-Al2O3(0.8 g),纯氨500 ℃为最佳条件,对苯二甲酸收率58.3C%,腈选择性92.3%。另外,4%的Ca(OH)2/Al2O3也适用于苯甲腈的生产。催化剂用量1.2 g,停留时间1.87 s,裂解温度650 ℃,纯氨(160 mL/min载气流量),苯甲腈的收率和选择性分别为30.4c%和82.6%。
详情可见:
1. LJ Xu, XW Na, LY Zhang, Q Dong, GH Dong, YT Wang, Zhen Fang*, Selective Production of Terephthalonitrile and Benzonitrile via Pyrolysis of Polyethylene Terephthalate (PET) with Ammonia Over Ca(OH)2/Al2O3 Catalysts, Catalysts, 9, 436; doi:10.3390/catal9050436 (2019).
2. LJ Xu, LY Zhang, H Song, Q Dong, GH Dong, X Kong, Zhen Fang*, Catalytic Fast Pyrolysis of Polyethylene Terephthalate Waste Plastic for the Selective Production of Terephthalonitrile under Ammonia Atmosphere, Waste Management, 92, 97–106 (2019).